1. Introduction
The Red Sea is a narrow inland sea separating the Arabian Peninsula, western Asia, from northeastern Africa. It extends northwest from the strait of Bab El-Mandeb to Suez, Egypt, for a distance of 1900 km. The maximum depth of the sea is 3040 m, and its maximum width is 350 km. The northern extremity is divided by the Sinai Peninsula into the gulfs of Suez and Aqaba. The Suez Canal connects the Red Sea with the Mediterranean Sea and Bab El-Mandeb connects it with the Gulf of Aden, an arm of the Arabian Sea. (
Edwards, 1987). The northern Red Sea is permanently stratified throughout the year, mixing depth is <100 m, a deep chlorophyll maximum is present year-round at about 80-100 m depth, and the euphotic depth is well beyond 100 m (Pätzold
et al., 2000). The Red Sea environments receive, either locally or more widely, a variety of stresses as a result of man’s activities. Considerable industrial and urban development's are currently taking place at many locations both onshore and offshore around the Egyptian Red Sea coast (
El-Tahera and Madkour, 2014).
The quality of water and its capacity to sustain heterotrophic communities, dynamic changes in pH, concentrations of oxygen and inorganic nutrients (nitrate, phosphate, ammonia) are all closely associated with fluctuations in phytoplankton composition (
Hulyal and Kaliwal, 2009).
Phytoplankton composition and abundance are considered as natural bio-indicators of water quality variations because of their sensitivity and rapid responses to changes in environmental conditions such as pH, light, temperature, salinity, turbidity and nutrients (
Stanca et al., 2013). Thus, the species compositions, relative abundance, spatial and temporal distribution of this aquatic biota are an expression of the environmental health or biological integrity of a particular water body.
Most knowledge on the phytoplankton population of the Red Sea is mainly derived from the review of
Halim (1969) who listed about 209 species (125 dinoflagellates and 84 diatoms) and
Skaikh et al. (1986) recorded 283 species (of them, 110 dinoflagellates and 137 diatoms).
Dowidar et al. (1978) recorded about 224 species classified as 111 dinoflagellates and 112 diatoms. Halim (1984) indicated that southern Red Sea is more productive than the northern area.
Nassar (1994, 2000),
Nassar and Hamed (2003),
Hamed et al. (2003),
Shams El-Din et al. (2005),
Deyab et al. (2004),
Ismail (2005) and
Nassar (2007a) examined the seasonal variations of phytoplankton at different locations in the Gulf of Suez. Several workers including
Gordon et al.
(1994),
Post et al.
(2002),
Al-Najjar et al. (2007) and
Nassar (2007b) studied the population density of phytoplankton in the Gulf of Aqaba,. Also,
Dorgham et al. (2012) concluded that the phytoplankton biomass in the Gulf of Aqaba was generally low in the epipelagic zone, whereas chlorophyll a was less than 0.5 μg/L, except relatively high concentration (0.7-1.12 μg/L) in deep layers in spring. In the Red Sea Proper,
El-Sherif and Abo El-Ezz (2000) examined the distribution of plankton at the northern Red Sea, recording 41 diatom species, 53 dinoflagellates, 10 cyanophytes and two chlorophytes.
Abel Rahman and Nassar (2005) studied the phytoplankton and zooplankton at the sector of Halayib-Shalatin, Red Sea.
Madkour et al. (2010) reported that the phytoplankton population was fairly diversified (181 species) and comprised mainly two groups; dinoflagellates (116 species) and diatoms (60 species).
Nassar et al. (2014) recorded 145 species of phytoplankton with clear dominance of Bacillariophyceae, which formed about 76.4 % of the total phytoplankton counts.
Nassar et al. (2015) indicated that the total abundance of phytoplankton was relatively low in the eastern coast of Suez Gulf, as compared its western coast and the northern part of the Red Sea.
Ismail (2015) conducted a review on the phytoplankton distribution in the whole Red Sea, recording 389 species and varieties.
Generally, about 207 phytoplankton species that identified and classified by many workers at different areas in the Egyptian waters of the Red Sea and some surrounding habitats are listed by
Nassar and Khairy (2014). A guide photos for most of these phytoplankton species are represented by
Nassar and Khairy (2015).
In fact, the available literatures on phytoplankton population dynamic at the different harbors of the Red Sea, Egypt are scarce and information is lacking concerning phytoplankton in this area. The aim of the present study is to follow up the impact of different activities and effect of nutrients on the spatial and temporal variations of phytoplankton at some of the Egyptian harbors of the Northern Red Sea.
2. Material and Methods
2.1 Description of sampling stations
Eighteen different stations were selected for investigation in the present study and covering about nine different harbors (
Fig.1). The stations 2, 3, 5, 9, 10, 12, 15 and 17 are located inside the harbors, while the stations 1, 4, 6, 8, 11, 13, 16 and 18 are selected outside the ports of the study area. The other two sites; St.7 is selected near Tersana area of Port-Tawfik Port and St.14 is situated near Al-Tour Maritime area. The chosen harbors could briefly describe as follows:
1- Ataka Fishing Port (St. 1, 2): Fishing port in Ataka 20 km south Suez. It has 2 berths and host about 150 fishing vessels within its basin.
2- Adabiya port (St. 3, 4). The Port is situated on the western coast of Suez Gulf at about 17 km from Suez City (32º 29 E and 29º 52 N). The harbour is open to the Suez Bay southern area and extends till the head of Adabiya region.
3- Zyteia Port (Petroleum Dock port) (St. 5, 6). The port is located on the Eastern part of Suez-port on the northern side of Suez Gulf at the Southern entrance of Suez Canal (32 31.8 E, 29 57.2 N). It has long basin where the tankers can dock for upload and download the petroleum oil.
4- Port Tawfik (St. 7, 8, 9). It is located on the northern part of Suez Gulf on the southern gate of Suez Canal. It includes the northern coast until Suez Canal entrance (32 34 E, 29 56 N). It has two basins, the first one for the shipping activity and the other for the maintenance of the ships (El Tersana, St.7).
5- Sukhna Port (St. 10, 11). It is a new commercial port, located on the western coast of Gulf of Suez and away from Suez city 43 km ( 32 21.4 E, 29 39 N). The port has 3 berths; two of them are working.
6- AL-Tour port (St. 12, 13). AL-Tour port is located on the eastern coast of Gulf of Suez with one berth (about 280 km southwards Suez city) (33 37 E, 28 14 N).
7- AL-Tour maritime fishing port (St.14). It is a fishing port Located on the eastern coast of Gulf of Suez, host from 75 to 80 fishing boat with a berth length of 75 m (33 60 E, 28 23 N).
8- Nuweiba port (St.15, 16). This port is located on the western coast of Aqaba Gulf at approximately 168 km North of Sharm El-Sheikh, and about 64 km. south of Taba (34 39 E, 28 57N).
9- Sharm El Sheikh Port (St.17, 18). The port is located about 380 km south of Suez Governorate. It is located on the top of the triangle of Sinai Peninsula, at the confluent of Suez and Aqaba Gulfs in South Sinai Governorate (34 17 E, 27 51.2 N).
.png)
Figure 1 Positions of the sampling stations
|
2.2 Physico-chemical parameters
Water temperature was measured by using a simple pocket thermometer graduated to 0.1
oC. The pH value of water samples was measured in situ using a pocket pH meter model Orion 210, Dissolved oxygen was fixed in field and measured according to the modified Winkler’s method according to
Strickland and Parson (1972) and the dissolved inorganic nutrients (NO
3, NO
2, NH
4 and PO
4) were determined spectrophotometrically and the results were expressed in µM according to the methods described by
APHA (2005).
2.3 Phytoplankton estimations
Surface water samples were seasonally collected from different 18 stations covering nine harbors along the two gulfs of the Red Sea during summer and autumn of 2014 and winter and spring of 2015. The community structure and standing crop of the phytoplankton was determined by sedimentation method (Ütermohl, 1958), and expressed in cell or unit per liter. The accepted and recent names of the recorded phytoplankton species were according to the taxonomic database sites, like algaebase.com (ab), World Register of Marine Species (WoRMS), Canadian Register of Marine Species (CaRMS), Nordic Microalgae and Aquatic Protozoa (NOD) and Integrated Taxonomic Information System (ITIS).
2.4 Statistical analysis
The correlation matrices was applied to total phytoplankton counts and the physico-chemical parameters at p< 0.05 and n=60. Multiple regressions were calculated for phytoplankton, using the program of STATISTICA Version 5. The species diversity (H′) was calculated according to
Shannon-Weaver (1963). This statistical analysis was carried out for only 15 sampling stations at which the phytoplankton samples were estimated in four seasons. But the stations 2 (not detected for phytoplankton in summer, 2014), 10 and 11 (not detected in winter, 2015) are excluded from the analysis.
3. Results and Discussion
3.1 Physico-chemical conditions
Water temperature is the most important environmental parameter, which affects the organisms and chemical and biological reactions in water (
Salah and El-Moselhy, 2015). In the present study, the highest seawater temperature 27.5 °C was found at St 11 (Sukhna out port) during summer of 2014, while the lowest value of 16.5 °C was recorded at St.5 (Zyteia in port) during winter of 2015. Generally, the maximum mean value of 26.11 °C was recorded during summer and the minimum mean value of 19.24 °C was observed in winter. An inverse correlation (r= - 0.57) was found between the seasonal variations of phytoplankton and water temperature.
pH is one of the vital environmental characteristics for the survival, metabolism, physiology, growth of aquatic organisms and chemical processes (
Ramanathan et al., 2005). It is controlled by the dissolved oxygen, water temperature, sewage discharge; decomposition of organic matter and photosynthetic activities (
Nassar and Hamed, 2003). Low pH values could allow toxic elements to become more available for uptake by aquatic plants and animals. This can produce conditions that are toxic to aquatic life (
Faragallah et al., 2009). In this study the relatively higher pH values were recorded during summer (8.11-8.27) in which the rise in temperature usually causing more consumption of carbon dioxide due to the phytoplankton photosynthetic activity. On the other hand, lower pH values were observed during winter (7.75-8.20) in which there is an increasing in CO
2 level because the increasing of the rate of organic matter decomposition by the microbial respiration.
Dissolved oxygen is an important constituent of water and its concentration in water is an indicator of prevailing water quality and ability of water body to support a well-balanced aquatic life (
George et al., 2012). Measure of dissolved oxygen refers to the amount of oxygen contained in water, and defines the living conditions for oxygen-requiring (aerobic) aquatic organisms (
Sundarambal et al., 2009). It is also the best parameter to show the effect of pollution in the aquatic ecosystem unless it contains toxic constituents (
Salah and El-Moselhy, 2015). The data in
Table 1 indicated that the highest DO values of 10.39 and 9.61 mgO
2/L were found at the stations 1 (Ataka out port) and 4 (Adabiya out port), respectively during winter of 2015. While, the lowest values of 3.35 and 4.13 mgO
2/L were detected at the stations 15 (Nuwbia in port) and 2 (Ataka in port), respectively during summer of 2014. Seasonally, lowest values of DO were recorded during summer (average of 5.9 mgO
2/L) in which the lowest abundance of phytoplankton was observed (average of 2821 unit/L), whereas the highest values of DO was recorded during winter (average of 8.45 mgO
2/L), which was associated with highest flourishing of phytoplankton (average 8269 unit/L). This high oxygen content in winter indicates a good mixing in the water column (
Girgis, 1980). However, the coastal waters require minimum value of oxygen 4.0 mgO
2/L and it will be better with 5.0 mgO
2/L for optimum ecosystem function (
UNESCO/WHO, 1973).
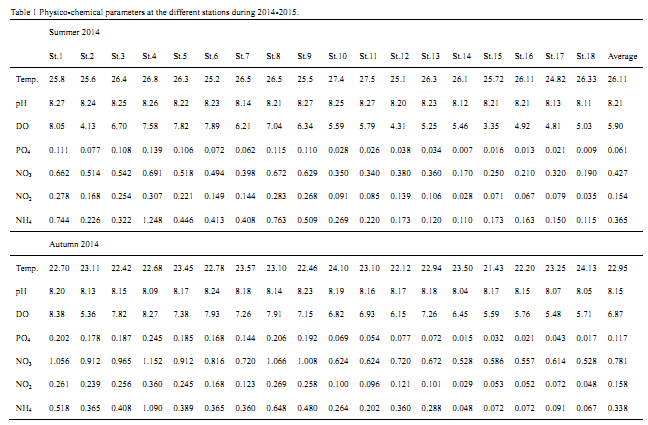
Table 1 Physico-chemical parameters at the different stations during 2014-2015.
|
Nutrients are considered critical besides water and carbon dioxide that are consumed by plants in the synthesis of organic materials (
Hamed and Said, 2000).The nutrient enrichment of coastal waters is generally the main factor driving the succession and composition of phytoplankton communities (
Leterme et al., 2014).The most important nitrogen sources for the phytoplankton growth are the nitrate and ammonium salts (
Nassar, 2014). Both EU and OSPAR Organizations defined the eutrophication specifically refers to the compounds of nitrogen and phosphorus of anthropogenic origin. It is widely accepted that nitrogen is the limiting nutrient for phytoplankton growth in marine waters and the nitrate-nitrogen is generally deemed to be the principal driver of eutrophication, while the phosphorus being the limiting nutrient in freshwaters (
Thie-Scroodie-Stoo and Yn Reiltys, 2006).
Ammonium is biologically active compound present in most water as normal biological degradation product of organic nitrogen (
Salah and El-Moselhy, 2015). Ammonium (NH
4+) represented 80% of Dissolved Inorganic Nitrogen (DIN) and its highest values were always associated with fresh water inflow (
Martin et al., 2008). Results in
Table 1 showed that, highest concentration of ammonium (1.248 µmole/l) was found during summer at station 4 (Adabiya out Port). This may be due to the effluents of partial treated sewage of ABB Company, and the impacts of fishing ships and other human activities near this area. While, the lowest value of 0.048 µmole/l was observed at St. 14 (Al-Tour maritime). Generally, ammonium contents were observed to be high at the relatively polluted stations 4, 8 and 1, during summer 2014 in which the lowest abundance of phytoplankton was occurred.
The consideration of Nitrite concentrations in seawater is useful due to its intermediate oxidation state between ammonia and nitrate, but it has the highest toxicological significance of human health, if present in perceptible concentration in diets (
Nassar and Hamed, 2003). Maximum nitrite concentration of (0.408umol/L) was recorded at St.4 (Adabia out of the port) during winter (
Table 1) where, water mixing reached its highest rate and nitrite accumulation in the water column is due to excretion by algal cells which resulted in more phytoplankton growth. Such value is in quite agreement with the presence of ammonia at the same station and during the same season. The minimum value of nitrite being (0.028umol/L) occurred at St.14 (Al-Tour maritime) during summer.
Nitrate is considered as the most stable and predominant inorganic nitrogen form in seawater and it is one of the main nitrogen sources for phytoplankton (
Al-Qutob et al., 2002). In the present study, dissolved nitrate represented about 57.32% of the total inorganic nitrogen. The maximum nitrate concentration of 1.262 µmole/l was observed during spring at St.4 (Adabiya out port), this was associated with high flourishing of total phytoplankton counts, while the minimum value of 0.170 µmole/l was recorded during summer at St.14. (Al-Tour maritime) as shown in
Table 1 and
Fig. 2. This result is achieved by a strong direct correlation between dissolved nitrate and the seasonal variations of phytoplankton (r=0.8).
.png)
Figure 2 Seasonal variations of physico-chemical parameters at the different stations during 2014-2015.
|
Phosphorus is an important element for the growth and primary production of phytoplankton in the aquatic systems. A large amount of it in the waters leads to eutrophication and becomes a potential source of pollution (
Nassar, 2014). As shown in
Table 1, the dissolved inorganic phosphate was fluctuated between a maximum of 0.413 µmole/l during winter at the relatively polluted St.4 and a minimum of 0.007 µmole/l during summer at St.14. High load of sewage and industrial wastes discharged into the Suez Bay may lead to gradual deterioration of water quality in the Bay. However, it was obvious that the Suez Bay (Stations 1-9) which is affected by partial treated sewage of ABB Company is considered as eutrophic area as compared with other sites of the study area in agreement with that of
Nassar and Hamed (2003) who stated that the Suez Bay is considered as eutrophic area.
3.2. Phytoplankton
3.2.1. Community composition
The phytoplankton community was represented by 146 species of phytoplankton belonging to 57 genera. These species are identified as 86 species of diatoms, 39 dinoflagellates, 15 cyanophytes, three of euglenophytes, two species of chlorophytes and one of silicoflagellates. The diatoms were the leading and most dominated group, forming about 81 % of the total counts of phytoplankton with total counts of 5353 unit/l, followed by dinoflagellates that represented about 14.04% of the total abundance with total counts of 928 unit/l. While, other algal classes were collectively formed about 4.96% of the total counts of phytoplankton (
Table 2).
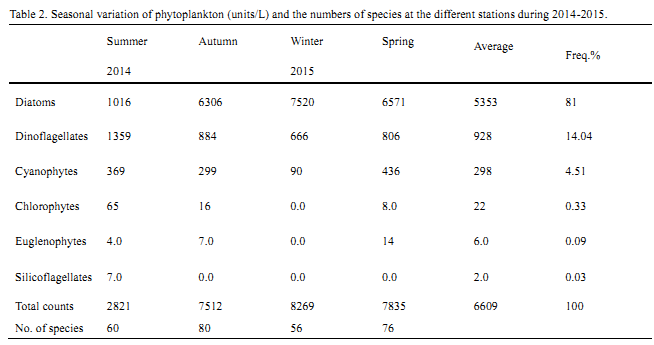
Table 2 Seasonal variation of phytoplankton (units/L) and the numbers of species at the different stations during 2014-2015.
|
The highest abundance of diatoms (86 species belonging to 33 genera) in the Egyptian harbors of the Red Sea was associated with the high flourishing of
Thalassionema nitzschioides (Grunow) Mereschkowsky that represented about 26% of the total diatoms and about 19.8% of the total phytoplankton during the period of study with annual average of 1389 cell/l.
Asterionellopsis glacialis (Castracane) Roundand
Proboscia alata var.
gracillima (Brightwell) Sündstrom were also dominant forming about 19.8 and 17.73% of the total diatoms with average of 1060 and 949 cell/l, respectively. While,
Rhizosolenia styliformis Brightwell (average of 215 cell/l),
Pseudo-nitzschia pungens (Grunow ex Cleve) G.R.Hasle (average of 215 cell/l) and
Chaetoceros tortissimus Gran (average of 213 cell/L) were frequently distributed at the different sites of the study area. Generally, the diatoms are usually dominated in the Red Sea, where it constituted about 76.4 % of the total abundance in the northwestern part of the Red Sea (
Nassar et al., 2014) and about and 67.00 % in the eastern coast of Suez Gulf (
Nassar et al., 2015).
The dinoflagellates in the present study (39 species belonging to 11 genera) were observed with moderate abundance with a relative high occurrence of
Ceratium furca (Ehrenberg) Claparède & Lachmann (average of 150 cell/l),
Ceratium fusus (Ehrenberg) Dujardin (average of 136 cell/l) and
Protoperidinium cerasus (Paulsen) (average of 122 cell/l). Whereas, the other algal classes include Cyanophyceae (4.51%), Chlorophyceae (0.33%), Euglenophyceae (0.09%) and Silicoflagellates (0.03%) were found in low counts during 2014-2015 at the most stations (
Table 2 &
Fig.3).

Figure 3 New ICT based fertility management model in private dairy farm India as well as abroad
|
The stations 4 (Adabiya out harbor) and 8 (Port-Tewfik out Harbor) were the most productive sites with total abundance of 51769 unit/l and 47283 unit/l, respectively, then followed by the stations 1 (Attaka out Harbor), 9 (Port-Tewfik inside Harbor), and 3 (Adabiya inside harbor) with total counts of 42508, 41103 and 39948 unit/l, respectively. This was associated with high flourishing of the previous dominant diatoms as well as a relative high occurrence of
Prorocentrum hentschelii Schiller of dinoflagellates at St.4. This result may be due to the partial treated sewage discharged from ABB Company into the waters of Suez Bay, which increases the eutrophication state of this area (
Nassar and Hamed, 2003). Also, the discharge of low fractions of petroleum hydrocarbons derived mainly from El-Nasr Petroleum Company and Suez Petroleum Company may stimulate the growth and reproduction of the phytoplankton cells (
Nassar, 1994). In this connection,
Burford et al. (2012) indicated that the low amounts of sewage may increase the phytoplankton productivity, and the low fractions of petroleum hydrocarbons could promote the phytoplankton growth (
Nayar et al., 2005).
On the other hand, the stations 16 (Nuwbia out Harbor), 17 (Sharm El-Sheikh inside Harbor) that located in the Gulf of Aqaba and St.14 (Al-Tour Maritime) that cited in the eastern coast of Suez Gulf were the lowest productive sites with total counts of 13115, 10589 and 10045 unit/l, respectively. This mainly may be due to low nutrients as reported by
Fahmy (2001, 2003),
Abel-Halim et al. (2007) and
Dorgham et al. (2012) who stated that the nitrogen, phosphorus and reactive silicate concentrations were generally low in the Gulf of Aqaba and the Egyptian Red Sea coastal water, which are classifying as oligotrophic to mesotrophic. In the present study, it was obvious that most of the high productive sites were observed out of the investigated harbors, may be due to low human and fishing impacts as well as low charging and discharging processes. However,
Ismail (2015) indicated that the phytoplankton of the Red Sea comprised 389 species and varieties, an increase of 181 species since Halim’s review in 1969. Both the Gulf of Suez and Gulf of Aqaba were less diverse than the main Red Sea. There is a gradual decrease in phytoplankton richness from the southern Red Sea to the Gulf of Suez. Primary production and chlorophyll biomass increases from north to south, consistent with the distribution of nutrients.
3.2.2. Seasonal variations of phytoplankton
The highest population density of phytoplankton was found during winter of 2015 with average counts of 8269 unit/l, then followed by spring of 2015 (average of 7835 unit/l) and autumn of 2014 (average of 7512 unit/l). While, a severe drop of phytoplankton abundance was observed in summer of 2014 (average of 2821 unit/l). This was in accordance with the data of
Nassar (2007a, b) in the Gulfs of Suez and Aqaba of the Red Sea, where the winter showed a high-pronounced peak of phytoplankton. While, severe drop of phytoplankton populations was observed in summer. Also,
Halim (1969),
Levanon-Spanier et al. (1979) and
Skaikh et al. (1986) stated that late winter-early spring or the period from December-February were characterized by high phytoplankton production in the Red Sea. On the other hand,
Nassar et al. (2014, 2015) reported that the autumn was the most productive season in the eastern coast of Suez Gulf and the northwestern part of the Red Sea.
The relative high abundance of phytoplankton during winter was associated with high flourishing of the diatoms;
Asterionellopsis glacialis (average of 3720 cell/l),
Thalassionema nitzschioides (average of 1404 cell/l),
Chaetoceros tortissimus (average of 714 cell/l),
Pseudo-nitzschia pungens (average of 516 cell/l) as well as a relative high occurrence of
Ceratium furca of dinoflagellates (average of 132 cell/l). This highest abundance of phytoplankton in winter was directly correlated with the high values of DO (average of 8.45 mgO
2/l) and nitrate (average of 0.822 µmole/l). However,
Rhizosolenia alata and
Pseudosolenia calcar avis appeared with high densities during winter in the Gulf of Suez, Red Sea (
Madkour et al., 2010). In autumn,
Proboscia alata var.
gracillima was the leading species forming about 42.5% of the total phytoplankton with maximum density at St.8 (total of 10000 cell/l). In spring,
Thalassionema nitzschioides represented about 52% of the total diatoms with maximum occurrence at St.4 (total of 13333 cell/l). On the other hand, the two dinoflagellates;
Protoperidinium cerasus and
Prorocentrum hentschelii showed their maximum abundance during summer (average of 202 and 201 cell/l, respectively), which sustained the lowest abundance of phytoplankton. Around the year, the annual average counts of phytoplankton was 6609 unit/l and the total number of species were 60, 80, 56 and 76 during summer, autumn, winter and spring, respectively as shown in
Table 3.
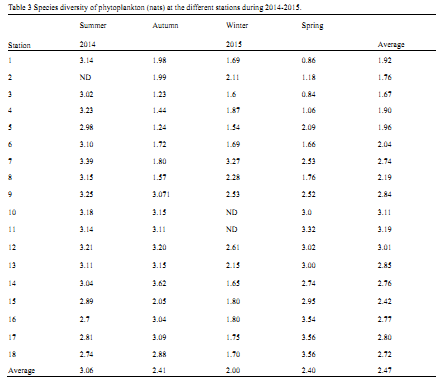
Table 3 Species diversity of phytoplankton (nats) at the different stations during 2014-2015.
|
In the present study some of the recorded species like,
Ceratium sp.,
Dinophysis sp.,
Protoperidinium sp.,
Prorocentrum sp.,
Oscillatoria sp. and
Dictyocha fibula are known to produce toxins that affect fish species (
Limbu and Kyewalyanga, 2015). The existence of these toxic phytoplankton species suggests careful consumption of fisheries resources at the different harbors of the study area and frequent monitoring for Harmful Algal Blooms (HABs) is required.
3.2.3. Species diversity
Species diversity is the number of different species that are represented in a given community (a dataset). The effective number of species refers to the number of equally abundant species needed to obtain the same mean proportional species abundance as that observed in the dataset of interest; where all species may not be equally abundant (
Tuomisto, 2010).
In the present study, the minimum diversity value of 0.84 nats was recorded during spring of 2015 at St.3, in which only 18 species of phytoplankton were found. This means a relative high pollution state inside Adabiya Harbor, which allowed the highest flourishing of the diatom,
Thalassionema nitzschioides to represent about 87% of the total phytoplankton at this area in spring, while other species were fairly distributed with low to moderate counts. On the other hand, the maximum diversity value of 3.62 nats was detected in autumn of 2014 at St.14 (Al-Tour Maritime) in which about 40 species was observed.
Halim (1984) reported that the species diversity of Red Sea plankton is reduced relative to the Indian Ocean, but much higher than that of the Arabian Gulf. However, the annual average diversity of 2.47 nats in this study is relatively low as compared with that recorded in the northwestern part of the Red Sea (3.76 nats;
Nassar et al., 2014) and in the eastern coast of Suez Gulf (3.22;
Nassar et al., 2015). However, the analysis of data in
Table 4 shows that most of the harbors that located in the northern part of Suez Gulf (Stations 1 to 9) are characterized by a relatively low values of species diversity as compared with the other investigated harbors in the Gulfs of Suez and Aqaba (Stations 10 to 18 that representing the Harbors of Ain Sukhna, Al-Tour, Nuweiba and Sharm El-Sheikh). These findings indicate that most of eastern coast of Suez Gulf and the western coast of Aqaba Gulf are still healthy, relatively unpolluted and have the oligotrophic to mesotrophic nature, as compared with other northern area of Suez Gulf.
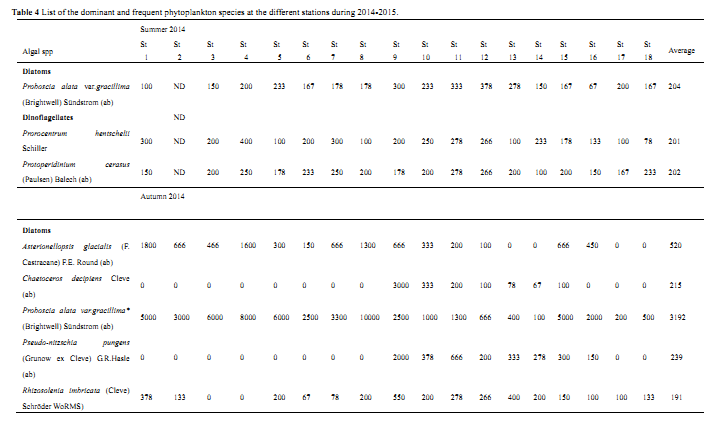
Table 4 List of the dominant and frequent phytoplankton species at the different stations during 2014-2015.
|
4. Statistical analysis
The correlation analysis indicated that the seasonal variations of phytoplankton was positively correlated with the dissolved nitrate (r=0.8) and the dissolved oxygen (r=0.55) but it was inversely correlated with water temperature (r=-0.57) at p< 0.05 and n=60. On the other hand, the stepwise multiple regression analysis revealed that mainly dissolved nitrate followed by water temperature and pH values were the most effective variables that controlled the seasonal fluctuations of phytoplankton at the different sites of the study area during 2014-2015. The regression equation = - 56774.7 + 0.624 (NO3) + 0.165 (pH) + 0.22 (DO) +0.25 (PO4) - 0.25 (Temp.) at p < 0.05 and n=60. This equation could be concern in the future studies to predict the total counts of phytoplankton in the northern harbors of the Red Sea.
Conclusion
The results indicated the highest abundance of phytoplankton was observed at the stations near the northern part of the Suez Gulf, including Adabiya out harbor, Port-Tewfik out Harbor, Attaka out Harbor, Port-Tewfik inside Harbor and Adabiya inside harbor. This result may be due to the partial treated sewage discharged from ABB Company, and low fractions of petroleum hydrocarbons derived mainly from El-Nasr Petroleum Company and Suez Petroleum Company may stimulate the growth and reproduction of the phytoplankton cells. Whereas, Nuwbia out Harbor, Sharm El-Sheikh inside Harbor that located in the Gulf of Aqaba and Al-Tour Maritime and situated in the eastern coast of Suez Gulf were the lowest productive sites. This mainly may be due to low nutrients in the Gulf of Aqaba and the Egyptian Red Sea coastal water, which are classifying as oligotrophic to mesotrophic nature. In the present study, it was found that most of the high productive sites were observed out of the harbors, may be due to low human and fishing impacts as well as low charging and discharging processes. The highest abundance of phytoplankton during winter, 2015 was associated with highest values of DO and nitrate. The species diversity of phytoplankton (annual average of 2.47 nats) showed its minimum during spring at Adabiya inside harbor, which may reflect a relative high pollution state at this area. However, the existence of some toxic phytoplankton species in the present study suggests careful consumption of fisheries resources at the different harbors of the study area and frequent monitoring for Harmful Algal Blooms (HABs) is required.
Abel Rahman N. S. and Nassar, M. Z., 2005, Preliminary studies on plankton communities associated with the mangrove forest habitats at Halayib-Shalatin Sector, Red Sea, Egypt. Pakistan Journal of Marine Sciences, 14(2): 133-144.
Abel-Halim A.M., Aboel-Khair E.M. and Fahmy M. A. 2007, Environmental assessment on the Aqaba Gulf coastal waters; Egypt. Egyptian Journal of Aquatic Research, 33(1): 1-14.
AL-Najjar T., Badran M. I., Richter C., Meyerthofer M. &Sommer U., 2007, Seasonal dynamics of phytoplankton in the Gulf of Aqaba, Red Sea. Hydrobiologia 279: 69-83.
Al-Qutob M., Hase C., Tilzer M. M. and Lazar B., 2002, Phytoplankton drives nitrite dynamics in the Gulf of Aqaba, Red Sea,” Marine Ecology Progress Series, 239, pp. 233–239.
APHA (American Public Health Association), 2005, Standard Methods for the Examination of Water &Wastewater, twenty first Ed. Washington.
George B. J.I., Kumar N. and. Kumar R.N., 2012, Study on the influence of hydro-chemical parameters on phytoplankton distribution along Tapi estuarine area of Gulf of Khambhat, India. Egyptian Journal of Aquatic Research 38, 157–170.
Deyab M. A., Khedr A. A., and EL-Naggar M. A., 2004, Phytoplankton distribution in relation to environmental factor along the Suez Canal and the Red Sea coast of Egypt. Algological Studies, 112, 123–140.
Dorgham M. M., Abdel-Aziz N. E., El-Deeb K. Z. and Okbah M. A., 2004, Eutrophication problems in the Western Harbour of Alexandria, Egypt. Oceanologia, 46 (1): 25–44.
Dorgham M.M., EL-Sherbiny M.M. and Hanafi M.H., 2012, Environmental properties of the southern Gulf of Aqaba, Red Sea, Egypt. Medit. Mar. Sci., 13/2: 179-186
Dowidar N. M., Raheem El-Din S. A. and Aleem A. A., 1978, Phytoplankton population in the region of Obhur (Jeddah, Saudi Arabia). Bull. Fac. Sci., K. A. Uni., Jeddah, 2: 271-292.
Edwards A.J., 1987, Red Sea, Pergamon Press, Oxford, pp. 45.
El-Sherif Z. M. and Abo El-Ezz S., 2000, Checklist of plankton of the northern Red Sea. Pakistan J. Marine Science, 9 (1&2): 61-78.
El-Tahera A. and Madkour H.A., 2014, Environmental and radio-ecological studies on shallow marine sediments from harbor areas along the Red Sea coast of Egypt for identification of anthropogenic impacts. Isotopes in Environmental and Health Studies, 50(1): 120–133,
http://dx.doi.org/10.1080/10256016.2013.826211
Fahmy M. A., 2001, Preliminary study on the hydrochemistry of the Egyptian coastal water of Aqaba Gulf, as a unique ecosystem during year 2000. Bull. Nat. Inst. Oceanogr. & Fish., ARE, 27: 95-112.
Fahmy M.A., 2003, Water quality in the Red Sea coastal waters (Egypt): Analysis of spatial and temporal variability. Chemistry and Ecology. 19(1): 67-77.
Faragallah H. M., Askar A. I., Okbah M. A. and Moustafa H. M., 2009, Physico-chemical characteristics of the open Mediterranean Sea water for about 60 Km from Damietta harbor, Egypt. Journal of Ecology and the Natural Environment, 1(5): 106-119.
Girgis A., 1980, Investigation of level and effects of pollutants on saline lakes and littoral marine environments. Part II: Hydrography of Lake Qarun. Scientific Report, p. 166.
Gordon N., Angel D. L., Neori A., Kress N. and Kimor B., 1994, Heterotrophic dinoflagellates with symbiotic Cyanobacteria and nitrogen limitation in the Gulf of Aqaba. Mar. Ecol. Prog. Ser., 107: 83-88.
Halim Y., 1969, Plankton of the Red Sea. Oceanogr. Mar. Biol. Ann. Rev., 7: 231-275
Halim Y, 1984, Plankton of the Red Sea and the Arabian Gulf. Deep Sea Research Part A. Oceanographic Research Papers, 31(6–8): 969–982.
Hamed M. A., and Said T. O., 2000, Effect of pollution on the water quality of the Gulf of Suez. Egypt J. Aquat. Biol. and Fish., 4 (1):161-178.
Hamed M. A., Said T. O., and Nassar M. Z., 2003, Effect of heavy metals and aromatic hydrocarbons on phytoplankton biomass as represented by chlorophyll-a in the northern part of the Suez Gulf, Egypt”. Journal of Egyptian Academic Society for Environmental Development (D-Environmental studies), 4 (1): 67-85.
Hase C., Stamber N., and Al-Qutob M., 2000, Primary production and its control by the light and nutrient regimes: a comparative study between the Gulf of Aqaba and the northern Red Sea. 44 (22), p. 1999.
Hulyal, S.B., Kaliwal, B.B., 2009. Dynamics of phytoplankton in relation to physico-chemical factors of Almatti reservoir of Bijapur District, Karnataka State. Environ. Monit. Assess. 153, 45–59.
Ismael A. A., 2015, Phytoplankton of the Red Sea. Chapter in book: The Red Sea. pp 567-583. DOI 10.1007/978-3-662-45201-1_32
Leterme S. C., Jendyk J. G., Ellis A. V., Brownb M. H., and Kildea T., 2014, Annual phytoplankton dynamics in the gulf saint Vincent, South Australia, in 2011. Oceanologia, 56(4): 757–778.
Levanon-Spanier J., Padan E. and Reiss Z., 1979, Primary production in adesert-enclosed sea - the Gulf of Aqaba, Red Sea. Deep-Sea Res., 26:673-685.
Limbu S. M. and Kyewalyanga M. S., 2015, Spatial and temporal variations in environmental variables in relation to phytoplankton composition and biomass in coral reef areas around Unguja, Zanzibar, Tanzania. SpringerPlus, 4:646. doi. 10.1186/s40064-015-1439-z
Madkour F. F., El-Sherbiny M. M. and Amer M. A., 2010, Phytoplankton population along certain Egyptian coastal regions of the Red Sea. Egyptian Journal of Aquatic Biology and Fisheries, 14(2): 95–109.
Martin G.D., Vijay J.G., Laluraj C.M., Madhu N.V., Joseph T., Nair M., Gupta G.V.M., and Balachandran K.K., 2008, Fresh water influence on nutrient stoichiometry in a tropical estuary, South west coast of India. Appl. Ecol. Environ. Res. 6, 57–64.
Nassar M. Z., 1994, Effect of oil pollution on the quantity and quality of phytoplankton in Suez Bay of the Red Sea. Master’s Thesis, Faculty of Science, Tanta University.
Nassar M. Z., 2000, Ecophysiological studies on phytoplankton along the western coast of Suez Gulf. Philosophy Doctor Thesis, Faculty of Science, Tanta University.
Nassar M. Z., 2007a, Species composition and distribution of phytoplankton in the western coast of Suez Gulf, Egypt. Egyptian Journal of Aquatic Research, 33(2):113–132.
Nassar M. Z., 2007b, Nutrients and phytoplankton distribution in the coastal waters of Aqaba Gulf, Red Sea, Egypt. Egyptian Journal of Aquatic Research, 33(2):133–151.
Nassar M. Z., 2014, Phytoplankton community structure in relation to some water characteristics in Temsah Lake, Suez Canal, Egypt. International Journal of Oceans and Oceanography, 8(2): 113-126.
Nassar M. Z., and Hamed M. A., 2003, Phytoplankton standing crop and species diversity in relation to some water characteristic of Suez Bay (Red Sea), Egypt. Egypt.J.Aquat. Biol., Fish, 7(3):25-48.
Nassar M. Z., and Khairy H. M., 2014, Checklist of phytoplankton species in the Egyptian waters of the Red Sea and some surrounding habitats (1990-2010). Journal of Annual Research & Review in Biology. 4(23): 3566-3585.
Nassar M. Z., Hamdy R. M., Khairy H. M., and Rashedy S. H., 2014, Seasonal fluctuations of phytoplankton community and physico-chemical parameters of the north western part of the Red Sea, Egypt. Egyptian Journal of Aquatic Research, 40(4): 395–403.
Nassar M. Z., and Khairy H.M., 2015, Guide Photos of Major Phytoplankton Species in the Egyptian Waters of the Red Sea. World Science and Research Publishing. ISBN: 978-600-94857-1-0
Nassar M. Z., Shams El-Din N. G. and Gharib S. M., 2015, Phytoplankton variability in relation to some environmental factors in the eastern coast of Suez Gulf, Egypt. Environ Monit Assess, 187(10): 1-18. Doi: 10.1007/s10661-015-4874-y
Panda S. S., Dhal N. and Panda C., 2012, Phytoplankton diversity in response to abiotic factors along Orissa coast, Bay of Bengal. Int J Environ Sci., 2:3–8
Pätzold J., Halbach P. E., Hempel G. and Weikert H., 2000, "Meteor-Berichte: Ostliches Mittelmeer-Nor dliches Rotes Meer 1999 Cruise No. 44. 00-3". Leitstelle METEOR: Institut fur Meereskunde der Universitat Hamburg, Hamburg
Post A. F., Dedej Z., Gottli R., Li H., Thomas, D. N., El-Naggar A., El-Gharabawi M. and Sommer U., 2002, Spatial and temporal distribution of Trichodesmium spp., in the stratified Gulf of Aqaba. Mar. Ecol. Prog. Ser., 239: 241-250.
Ramanathan N., Padmavathy, P., Francis T., Athithian, S. and Selvaranjitham, N., 2005, Manual on polyculture of tiger shrimp and carps in freshwater,” Tamil Nadu Veterinary and Animal Sciences University, Fisheries College and Research Institute, Thothukudi, pp 1–161.
Redfield A. C., Katchum B. H. and Richards, S. A., 1963, The influence of organism on the composition of seawater, In the sea, vol. 2 M. N. Hill (Ed.), John Wiley and Sons, New York, pp. 26-77.
Salah, A. A. and El-Moselhy, K. M., 2015, Seasonal Variations of the Physical and Chemical Properties of Seawater at the Northern Red Sea, Egypt. Open Journal of Ocean and Coastal Sciences, 2(1): pp 1-17. Doi: 10.15764/OCS.2015.01001.
Shaikh, E.A.; Roff, J. C. and Dowidar, N. M., 1986, Phytoplankton ecology and production in the Red Sea off Jeddah, Saudi Arabia. Mari Biol., 92: 405- 416.
Shams El-Din, N. G., Nassar, M. Z. and Abd El Rahman, N. S., 2005, Surveillance studies on plankton in the northern part of the Red Sea during winter and summer, 2002. Journal of Egyptian German Society of Zoology. Vol. (48D): Invertebrate Zoology & Parasitology: 49-77.
Shannon, C.E. & Weaver, W., 1963, The mathematical theory of communications, Univ. Illinois, Urbana, 117 pp.
Strickland, J.D.H. & Parsons, T.R., 1972, A Practical Handbook of Seawater Analysis. Bulletin. Vol. 167, Ottawa, Canada: Fisheries Research Board of Canada, p. 310.
Sundarambal, P.; Shi-Yui, L.; Pavel, T. and Jegathambal, P., 2009, Development of a neural network model for dissolved oxygen in seawater. Indian Journal of Marine Sciences, 38(2): 151-159.
Thie-Scroodie-Stoo and Yn Reiltys, 2006, Environmental monitoring in Manx coastal waters: the Isle of Man time series. Governmental Report, Ballakermeen Road, Douglas, Isle of Man, IM1 4BR Website: www.gov.im/dlge/enviro/govlabs.
Tuomisto, H., 2010, A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography, 33, 2-22.
Doi:10.1111/j.1600-0587.2009.05880.x
UNESCO/WHO, 1973, Water quality surveys. A guide for the collection and interpretation of water quality data. Vol. 23, p. 350.
Utermöhl, H., 1958, Zur Vervollko¨mmung der quantitative Phytoplankton-Methodik. Mitteilungen Internationale Vereinigung fu¨ rtheoretische und angewandte Limnologie, 9, 1–38.
 Author
Author  Correspondence author
Correspondence author
.png)

.png)






